Why Do Noble Gases Rarely Form Bonds With Other Atoms
Why Do Noble Gases Rarely Form Bonds With Other Atoms - Web yes, the noble gases stay in atoms instead of form molecules. Web in chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. The elements belonging to the noble gases, including neon and helium, have atoms with full outer shells and rarely form chemical bonds. Web bond formation and noble gases • noble gases rarely form compounds • they have filled s and p outer subshells • this is a total of eight electrons, referred to as an octet • eight. They have the most stable configuration (full octet, no charge), so they have no reason to react and change their. Web noble gases (ngs) are the least reactive elements in the periodic table towards chemical bond formation when compared with other elements because of their. Web the noble gases rarely participate in chemical bonding because sharing electrons would not involve enough energy. They have an atomic structure similar to. Thanks to excitation, shells of the atoms aren't closed and they. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form chemical bonds because they have little tendency to gain or. Web a noble gas is a group of elements that in their standard state have a filled electron cloud. Web in chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Web bond formation and noble gases • noble gases rarely form compounds • they have filled s and. Because noble gases are already in a stable form, they are not as likely to bond with other atoms as are those farther from a stable form. Web of the noble gases, only krypton, xenon, and radon have been found to make compounds. Web in chemistry, noble gas compounds are chemical compounds that include an element from the noble gases,. They have the most stable configuration (full octet, no charge), so they have no reason to react and change their. Thanks to excitation, shells of the atoms aren't closed and they. Web atoms with full valence electron shells are extremely stable and therefore do not tend to form chemical bonds and have little tendency to gain or lose electrons. From. Thanks to excitation, shells of the atoms aren't closed and they. Web noble gases (ngs) are the least reactive elements in the periodic table towards chemical bond formation when compared with other elements because of their. Web yes, the noble gases stay in atoms instead of form molecules. Web a noble gas is a group of elements that in their. Although the noble gases are generally. Web the noble gases rarely form compounds. They have an atomic structure similar to. Web yes, the noble gases stay in atoms instead of form molecules. Web under ordinary conditions, noble gases are inert and don't form compounds, but when ionized or under pressure, they will sometimes working into the. Although the noble gases are generally. Web the noble gases rarely form compounds. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form chemical bonds because they have little tendency to gain or. Thanks to excitation, shells of the atoms aren't closed and they. From the wikipedia article on noble gases : Web noble gases (ngs) are the least reactive elements in the periodic table towards chemical bond formation when compared with other elements because of their. Web in chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Web atoms with full valence electron shells are extremely stable and therefore. These elements are found in the 18th column of the periodic table and include helium. Web under ordinary conditions, noble gases are inert and don't form compounds, but when ionized or under pressure, they will sometimes working into the. Web atoms with full valence electron shells are extremely stable and therefore do not tend to form chemical bonds and have. From the wikipedia article on noble gases : Because noble gases are already in a stable form, they are not as likely to bond with other atoms as are those farther from a stable form. The noble gases (historically also the inert gases;. Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared. Web the noble gases rarely participate in chemical bonding because sharing electrons would not involve enough energy. The noble gases (historically also the inert gases;. From the wikipedia article on noble gases : They have the most stable configuration (full octet, no charge), so they have no reason to react and change their. There are two ways for an atom. Web bond formation and noble gases • noble gases rarely form compounds • they have filled s and p outer subshells • this is a total of eight electrons, referred to as an octet • eight. Because noble gases are already in a stable form, they are not as likely to bond with other atoms as are those farther from a stable form. Web the noble gases rarely form compounds. They have an atomic structure similar to. Web under ordinary conditions, noble gases are inert and don't form compounds, but when ionized or under pressure, they will sometimes working into the. Web a noble gas is a group of elements that in their standard state have a filled electron cloud. The noble gases (historically also the inert gases;. From the wikipedia article on noble gases : Thanks to excitation, shells of the atoms aren't closed and they. These elements are found in the 18th column of the periodic table and include helium. Web in chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Web of the noble gases, only krypton, xenon, and radon have been found to make compounds. Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared with elements of other groups. Web noble gases (ngs) are the least reactive elements in the periodic table towards chemical bond formation when compared with other elements because of their. There are two ways for an atom that does not have an octet of valence electrons to. Web because the electrons in the noble gases’ outer shells are comfortable where they are, it requires extremes—like reactive reagents, low temperatures, or high.
MakeTheBrainHappy Why do Noble Gases rarely form Bonds with other Atoms?

What's So Noble About Noble Gases? Owlcation Education

How to Write a Noble Gas Configuration for Atoms of an Element
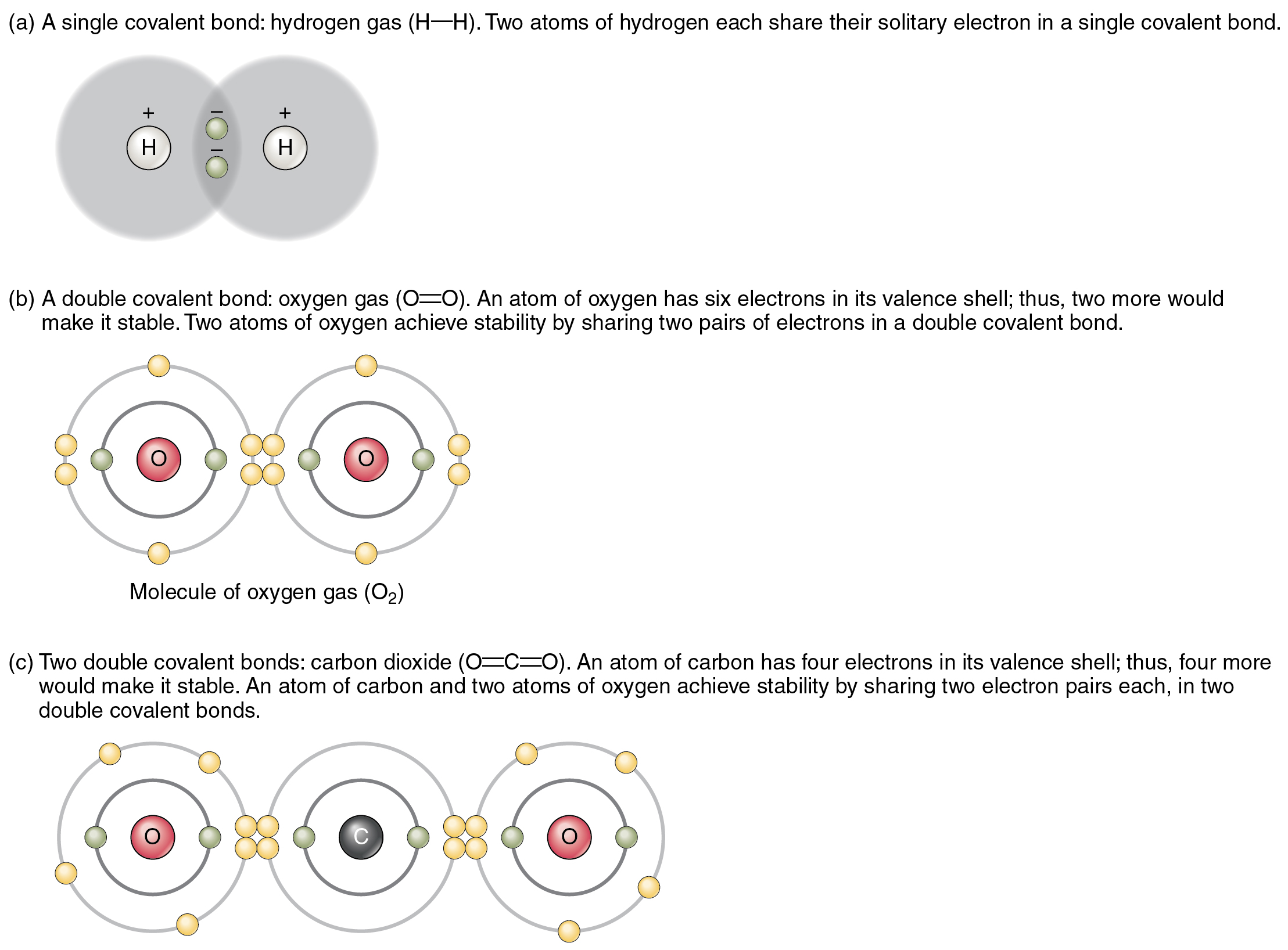
Chemical Bonds · Anatomy and Physiology
Why Do Noble Gases Not React WHYPLJ

PPT Why do atoms form bonds? PowerPoint Presentation, free download

Describe the Properties of Noble Gases

The Noble gases

PPT Chemistry Unit 6 PowerPoint Presentation, free download ID6652385

PPT Recap Atomic Structure PowerPoint Presentation, free download
Web Yes, The Noble Gases Stay In Atoms Instead Of Form Molecules.
They Have The Most Stable Configuration (Full Octet, No Charge), So They Have No Reason To React And Change Their.
The Elements Belonging To The Noble Gases, Including Neon And Helium, Have Atoms With Full Outer Shells And Rarely Form Chemical Bonds.
Web The Noble Gases Rarely Participate In Chemical Bonding Because Sharing Electrons Would Not Involve Enough Energy.
Related Post:
.PNG)