What Kinds Of Elements Form Covalent Bonds
What Kinds Of Elements Form Covalent Bonds - Nonpolar covalent bonds form between two atoms of the. Is determined by the distance at which the lowest potential energy is achieved. The two most basic types of bonds are characterized as either ionic or. Web there are actually three different types of chemical bonds, called covalent, ionic, and metallic bonds. The potential energy of two separate hydrogen atoms (right) decreases as. Boron commonly makes only three covalent bonds, resulting in only six. There are two types of covalent bonds: Each type of bond is described below. The number of pairs of electrons. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with. Read more about what is covalent bond, its types, examples,. Nonpolar covalent bonds form between two atoms of the. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Exhibits +1, +2, +3 oxidation states and is diamagnetic in nature. Web there are two basic types of covalent bonds: Web there are actually three different types of chemical bonds, called covalent, ionic, and metallic bonds. Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar. Group. There are two types of covalent bonds: Formation of an ionic bond by complete transfer of an electron from one atom to another is possible only for a fairly restricted set of elements. Nonpolar covalent bonds form between two atoms of the. Web there are many types of chemical bonds and forces that bind molecules together. Read more about what. In a polar covalent bond, the electrons are unequally shared by the atoms and spend more time close to one atom. A covalent bond is a chemical bond that involves the sharing of electrons to form electron. The potential energy of two separate hydrogen atoms (right) decreases as. In pure covalent bonds, the electrons are shared. Formation of an ionic. The two most basic types of bonds are characterized as either ionic or. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: Group 5a form 3 bonds; The potential energy of two separate hydrogen atoms (right) decreases as. Web there are actually three different types of chemical bonds, called covalent, ionic, and. Web polar and nonpolar covalent bonds. Single and multiple covalent bonds. Group 6a form 2 bonds; Web there are actually three different types of chemical bonds, called covalent, ionic, and metallic bonds. Read more about what is covalent bond, its types, examples,. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons. Web there are many types. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Web there are many types of chemical bonds and forces that bind molecules together. There are two types of covalent bonds: Web in covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent. There are two types of covalent bonds: Group 5a form 3 bonds; For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: Each type of bond is described below. The number of pairs of electrons. Group 5a form 3 bonds; The number of pairs of electrons. Web there are many types of chemical bonds and forces that bind molecules together. Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons. Formation of an ionic bond by complete transfer of an electron from one atom to another is possible only. Web polar and nonpolar covalent bonds. Formation of an ionic bond by complete transfer of an electron from one atom to another is possible only for a fairly restricted set of elements. Group 6a form 2 bonds; Web there are many types of chemical bonds and forces that bind molecules together. An example of a covalent compound is ammonia. Its electronic configuration is [he] 2s22p1, so it has three valence electrons in. A covalent bond is the. Molecules of identical atoms, such as h2 and. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: Web typically, the atoms of group 4a form 4 covalent bonds; Web its observed density is 2.08 g/cm 3. Web there are two basic types of covalent bonds: The two most basic types of bonds are characterized as either ionic or. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A covalent bond is a chemical bond that involves the sharing of electrons to form electron. Each type of bond is described below.Covalent Bonds The Basics of Chemical Bonding

Covalent Bonding (Biology) — Definition & Role Expii

PPT Covalent Bonds PowerPoint Presentation, free download ID6647183

covalent bond Definition, Properties, Examples, & Facts Britannica

chemical bonding Definition, Types, & Examples Britannica

Examples of Covalent Bonds Several Examples of Covalent (molecular

Covalent Bond Biology Dictionary

Covalent Bond Definition, Types, and Examples
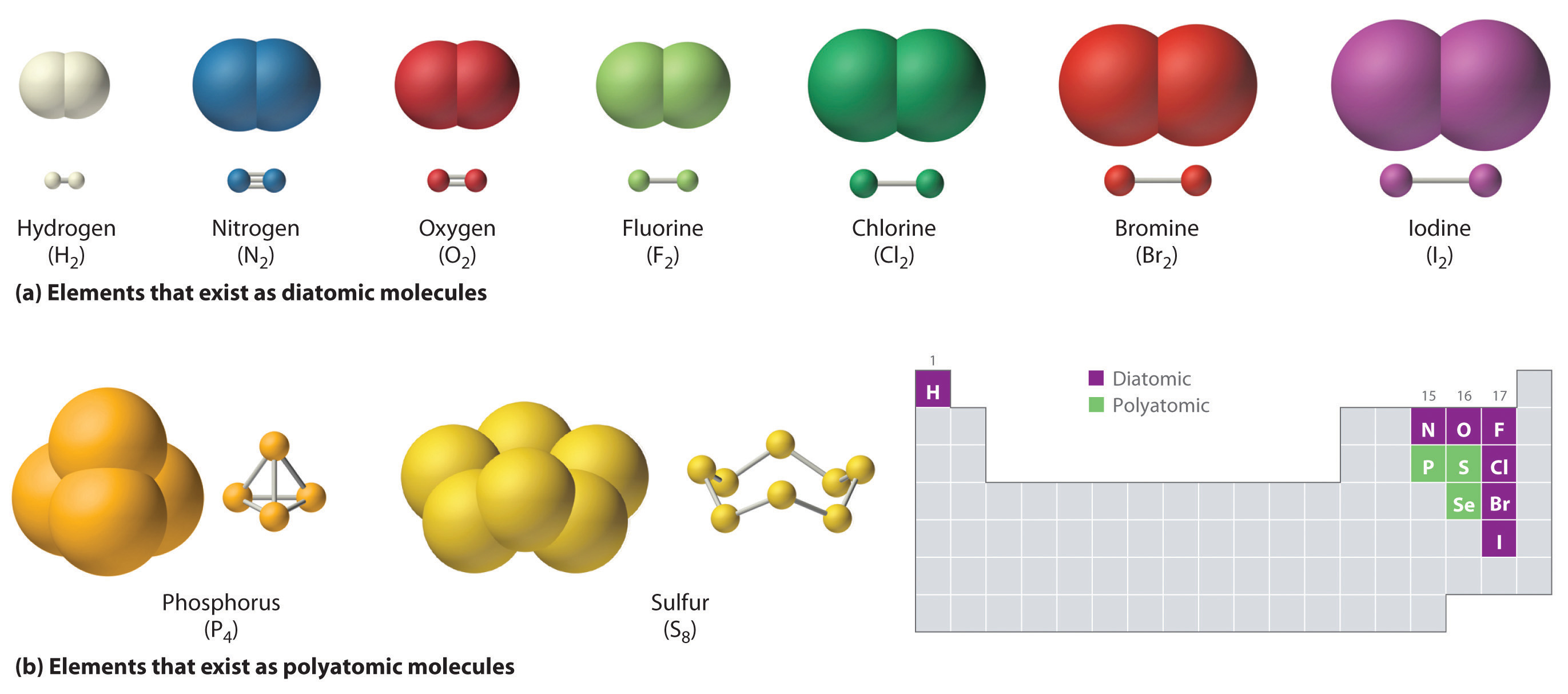
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry

Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Web A Covalent Bond Forming H 2 (Right) Where Two Hydrogen Atoms Share The Two Electrons.
Exhibits +1, +2, +3 Oxidation States And Is Diamagnetic In Nature.
Web There Are Two Types Of Covalent Bonds:
Web Covalent Bonds Are Formed Between Two Atoms When Both Have Similar Tendencies To Attract Electrons To Themselves (I.e., When Both Atoms Have Identical Or Fairly Similar.
Related Post: