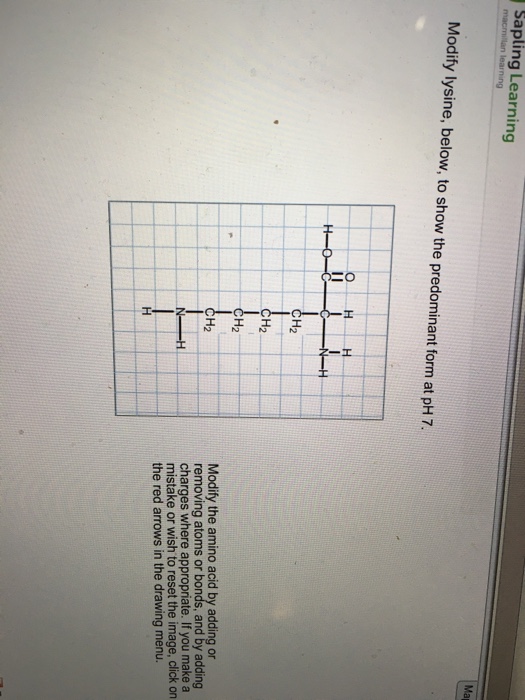
Modify Lysine To Show The Predominant Form At Ph 7
Modify Lysine To Show The Predominant Form At Ph 7 - Web the ph 7, suggests neutral state, that is, no proton in the solution. Modify lysine, below, to show the predominant form at ph 7. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Web modify lysine, below, to show the predominant form at ph 7. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Web modify lysine to show the predominant form at ph 7. Web modify lysine, below, to show the predominant form at ph 7. Web so since it's less, i mean, it's more acidic, which means that the protein ated form should predominate, and the protein ated form of a mean is an nh three. Web modify lysine, below, to show the predominant form at ph 7. Modify lysine, below, to show the predominant form at ph 7. Web the ph 7, suggests neutral state, that is, no proton in the solution. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Deduce the most acidic hydrogen in the given. Web draw structures of the predominant forms of lysine and aspartic acid at ph 3.0 and ph 9.7. Modify lysine, below,. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. At ph = 7, lysine possess net charge of. Modify lysine, below, to show the predominant form at ph 7. Modify lysine, below, to show the predominant form at ph 7. Web modify lysine, below, to show the predominant form at ph 7. At ph = 7, lysine possess net charge of. Modify the amino acid by adding or removing atoms or bonds and by adding charges where. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Web. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Modify lysine, below, to show the predominant form at ph. At ph = 7, lysine possess net charge of. Modify lysine, below, to show the predominant form at ph 7. Web question 6 of 25 > modify lysine to show the predominant form. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Modify the amino acid by adding or removing atoms or. Web draw structures of the predominant forms of lysine and aspartic acid at ph 3.0 and ph 9.7. Web modify lysine, below, to show the predominant form at ph 7. Modify the amino. Modify lysine, below, to show the predominant form at ph 7. Modify the amino acid by adding or removing atoms or bonds and by adding charges where. Web modify lysine to show the predominant form at ph7. Modify lysine, below, to show the predominant form at ph 7. Web draw structures of the predominant forms of lysine and aspartic acid. Web so since it's less, i mean, it's more acidic, which means that the protein ated form should predominate, and the protein ated form of a mean is an nh three. Web draw structures of the predominant forms of lysine and aspartic acid at ph 3.0 and ph 9.7. At ph = 7, lysine possess net charge of. Modify lysine,. 1000/1300 resources hint etion 8 of 13 > modify lysine to show the predominant form at ph 7. Web modify lysine to show the predominant form at ph7. Web question 6 of 25 > modify lysine to show the predominant form at ph 7. Modify the amino acid by adding or removing atoms or bonds and by adding charges where.. Web modify lysine, below, to show the predominant form at ph 7. Glutamic acid, leucine, arginine, histidine, & tyrosine. Modify lysine, below, to show the predominant form at ph 7. Modify the amino acid by adding or. Modify lysine, below, to show the predominant form at ph 7. Deduce the most acidic hydrogen in the given. Web draw the structure of the predominant form of (a) isoleucine at ph 11 (b) proline at ph 2 (c) arginine at ph 7 (d) glutamic acid at ph 7 (e) a mixture of alanine, lysine, and. Web so since it's less, i mean, it's more acidic, which means that the protein. Web predict the predominant ionized forms of the following amino acids at ph 7: 1000/1300 resources hint etion 8 of 13 > modify lysine to show the predominant form at ph 7. Web modify lysine to show the predominant form at ph7. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Modify the amino acid by adding or removing atoms or bonds, and by adding charges where. Web modify lysine to show the predominant form at ph 7. Web ma learning modify lysine, below, to show the predominant form at ph 7. Modify lysine, below, to show the predominant form at ph 7. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. As more base is added (ph increases), some of the. Modify the amino acid by adding or. Web starting at very low ph, the predominant structure of lysine is the dicationic form (refer to image above). Web draw structures of the predominant forms of lysine and aspartic acid at ph 3.0 and ph 9.7. Web modify lysine, below, to show the predominant form at ph 7. Modify lysine, below, to show the predominant form at ph. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate.Solved Modify lysine, below, to show the predominant form at

Modify lysine, below, to show the predominant form at pH 7.

Modify lysine, below, to show the predominant form at pH 7. Quizlet

Solved Modify lysine to show the predominant form at pH 7.

Solved Modify isoleucine to show the predominant form at pH
Solved Modify lysine, below, to show the predominant form at
Solved Question 12 Of 28 Ma Sapling Modify Lysine, Below,...

Solved Knowing the information found in the table below,

Solved Question 2 The structure of Lysine in the form

SOLVED aatsno Resources Hint Check Modify lysine to show the
Modify The Amino Acid By Adding Or Removing Atoms Or Bonds And By Adding Charges Where.
A) A Weak Base To Buffer.
Deduce The Most Acidic Hydrogen In The Given.
Modify Lysine, Below, To Show The Predominant Form At Ph 7.
Related Post: