How Many Covalent Bonds Does Phosphorus Form
How Many Covalent Bonds Does Phosphorus Form - Therefore the maximum number of covalent bonds. Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. How many valence electrons are present in the atom al? In each case, the sum. When phosphorus burns in chlorine both are. Web in both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be relatively strain free. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. The potential energy of two separate hydrogen atoms (right) decreases as. Values are given for typical oxidation number and coordination. Web how many bonds does phosphorus typically make? The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its. And group 7a form one bond. How many bonds does phosphorus typically make? Group 6a form 2 bonds; And group 7a form one bond. Web in both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be relatively strain free. Web there are many different modifications of phosphorus in nature. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other. Values are given for typical oxidation number and coordination. Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds. How many valence electrons are present in the atom al? Web there are many different modifications of phosphorus in nature. Web select all statements that correctly describe the typical number of covalent bonds formed. How many bonds does phosphorus typically make? Group 5a form 3 bonds; How many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom? Group 5a form 3 bonds; Group 6a form 2 bonds; Group 6a form 2 bonds; Web covalent radius half of the distance between two atoms within a single covalent bond. Web covalent bonds involve the sharing of electron pairs between atoms. The covalent bonds involve sharing of valence electrons between two atoms. Web typically, the atoms of group 4a form 4 covalent bonds; Web how many bonds does phosphorus typically make? How many valence electrons are present in the atom al? Web how does phosphorus form 5 covalent bonds? When phosphorus burns in chlorine both are. How many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom? How many bonds does phosphorus typically make? If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. Web in both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be relatively. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its. Covalent bond two atoms form a covalent chemical. Web select all statements that correctly describe the typical number of covalent bonds formed by common neutral atoms. Group 5a form 3 bonds; The potential. Similarly, the phosphorus atom in \(\ce{pcl_5}\) has 10 valence. When phosphorus burns in chlorine both are. Web typically, the atoms of group 4a form 4 covalent bonds; How many valence electrons are present in the atom al? Group 5a form 3 bonds; The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its. Covalent bond two atoms form a covalent chemical. How many valence electrons are present in the atom al? Web how many bonds does phosphorus typically make? This is summarized in the table below. Web covalent radius half of the distance between two atoms within a single covalent bond. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. Web how does phosphorus form 5 covalent bonds? Group 5a form 3 bonds; Group 5a form 3 bonds; How many valence electrons are present in the atom al? Web select all statements that correctly describe the typical number of covalent bonds formed by common neutral atoms. Web there are many different modifications of phosphorus in nature. And group 7a form one bond. Web meallic elements can definiely have more than eight valence electrons, however they do not tend to form covalent bonds. Web typically, the atoms of group 4a form 4 covalent bonds; Atoms with 5 valence electrons typically. The potential energy of two separate hydrogen atoms (right) decreases as. Web how many bonds does phosphorus typically make? The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.
How does phosphorus form 5 covalent bonds? The Unconditional Guru

Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
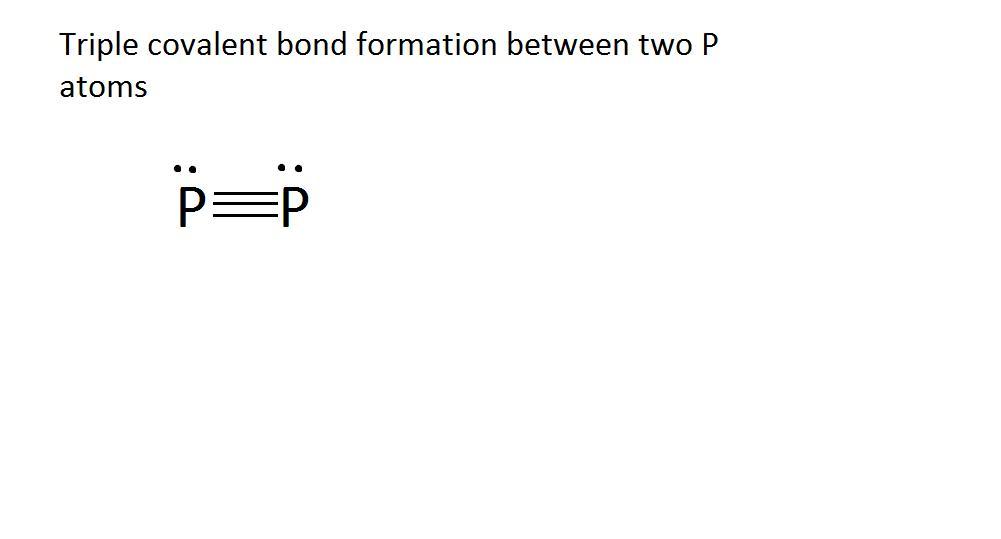
What type of bond would form between two atoms of phosphorus? A. Triple

Periodic Table Phosphorus Valence Electrons Periodic Table Timeline
41. What is the Lewis dot structure of phosphate ion? how many

covalent bond Definition, Properties, Examples, & Facts Britannica

How Many Valence Electrons Are Found In Phosphorus

Phosphorus Electron Configuration (P) with Orbital Diagram

Chapter 8 Covalent Bonding Covalent bonding Usually forms

What Is Covalent Bonding?
Therefore The Maximum Number Of Covalent Bonds.
Group 6A Form 2 Bonds;
And Group 7A Form One Bond.
How Many Covalent Bonds Does A Phosphorus Atom Normally Form, Based On The Number Of Valence Electrons In The Atom?
Related Post: